There are many crystals around us. Crystals are used in electronic devices such as cellular phones and computers, as well as daily accessories. Crystals are a very useful type of matter, and play a key role in industry. When it comes to the most familiar crystals, you probably think of snow crystals, or ice crystals in the freezer. Ice in the freezer looks like a clear and colorless mass, but if you wish, it's possible to make dendritic ice crystals with elegant symmetric patterns, just like snow crystals. Also, like minerals and proteins, some crystals are polyhedral, with flat surfaces like dice (Figure 1).
You might think that crystals are born with beautiful shapes. But the fact is that a very small crystal forms where nothing existed before, and it develops beautiful dendritic or polyhedral patterns over time. This phenomenon is called crystal growth, and the process is akin to the growth of a living thing.
Dendritic crystals have complicated patterns that look like tree branches. Ice crystals are the typical example. Our aim is to understand the formation of such crystalline shapes, and in order to do so, we need to closely observe crystal growth and understand its mechanisms. But unfortunately, on Earth the process of crystal growth is affected by fluid flow that occurs around the crystal. To avoid this, we need to do our experiments in an environment where the crystal's growth is completely undisturbed. This is why space is an ideal environment - no gravity, no fluid flow. We can achieve a level of precision that's impossible on the ground.
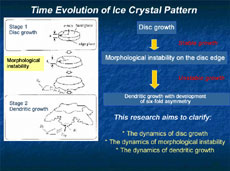
Space experiments in the Japanese Experiment Module Kibo will start in 2008, and the ice crystal experiment we proposed is one of them. For this experiment, we've developed a new observation device that automatically grows ice crystals and observes their development: first, a thin disc-shaped crystal is formed, and as it continues developing over time, it finally becomes a dendritic crystal with six-fold symmetry. The main objective of our space experiment is to understand specifically how an ice crystal transforms from a disc shape to dendritic growth. This phenomenon is called morphological instability, and we'll try to understand its dynamics.
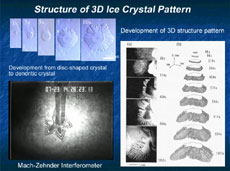
Ice crystals are very thin, like flower petals, so it had been presumed that their shapes were two-dimensional, and they had been studied based on this assumption. However, their thickness does, in fact, measure 1/10 or 1/20 of a millimeter, and this thickness must be taken into account. So we introduced a new method for the precise measurement of the thickness of crystals, and the concavity and convexity of their surfaces. This uses optical interferometry, which makes use of the subtle aberration of reflected laser beams. This has revealed that the edge of a disc-shaped crystal first expands like a skirt, and finally forms dendritically (Figure 2 and 3). This pattern development indicates that, with a disc crystal of ice, its round surface and side surface grow completely differently. Why does the same ice crystal have different growth mechanisms between the round surface and the side surface? How does it impact on the development of the crystalline patterns? There are endless interesting questions.
In 1998, the world's first space experiment with ice crystal growth was conducted using a sounding rocket launched by NASDA (now part of JAXA). This experiment, which used a microgravity environment created by the rocket's sub-orbital flight, confirmed the performance of the device we had designed for producing ice crystals (Figure 4). Unfortunately, we couldn't make ice crystals more than once because we were able to create the gravity-free environment for only 360 seconds. But our new device, which is an upgraded version, is now complete, awaiting its launch in 2008. The experiment will start in spring 2009. In the permanent microgravity environment of the International Space Station, it's possible to conduct the same experiment 100 times, or even 200 times. A great advantage of space experiments is the ability to repeat experiments over and over in such an ideal environment.
Crystalline patterns are a result of the growth of a crystal from birth, so to understand crystalline patterns, we must understand the mechanisms of crystal growth. Crystals are exceptionally elegant forms of matter with beautiful symmetry and an infinite variety of shapes. Snow and ice crystals are the most familiar to us, yet they are also among the most visually stunning. With these crystals, it is my long-time dream to unveil the universal secrets of crystal growth. This will contribute to the production of new types of crystals, which will also benefit human beings. I'm anticipating great results.
Yoshinori Furukawa
Professor, Institute of Low Temperature Science, Hokkaido University
Dr. Furukawa received his Ph.D. from the Graduate School of Science at Hokkaido University in 1978. He was appointed to his current position after working as a research assistant and an assistant professor at the Institute of Low Temperature Science at Hokkaido University.
Professor, Institute of Low Temperature Science, Hokkaido University
Dr. Furukawa received his Ph.D. from the Graduate School of Science at Hokkaido University in 1978. He was appointed to his current position after working as a research assistant and an assistant professor at the Institute of Low Temperature Science at Hokkaido University.