Q. What is the advantage of conducting protein crystal growth experiments in microgravity?

Ground-grown protein crystals forming in cluster (crystals are attached to each other)

Space-grown protein crystals are cleanly and discretely crystallized
Proteins are assembled from amino acids based on information encoded in genes, and each protein has a unique function. The human body contains more than 100,000 kinds of proteins; about 10 billion kinds exist in nature. Proteins are the basic building blocks of life.
Everyone has heard of DNA. DNA is mere information, synthesized with nucleic acid (the smallest molecules that make up DNA). Simply put, DNA is the blueprint of life. The blueprint dictates the types, number, and arrangement of molecules that make up proteins. It tells various proteins, such as keratin for hair and collagen for skin, how to function in our bodies. Proteins have a three-dimensional structure, and their shape plays a critical role in their function. So, to understand protein function, it is essential to study protein structure. With today’s technology, the three-dimensional structure of a protein can be revealed by collecting protein molecules to form a crystal and observing it by X-rays diffraction.
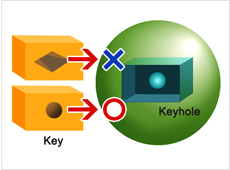
A protein does not work in isolation; it only carries out its characteristic function when a chemical reaction takes place. This is why, to understand protein functions, it is critical to look at what type of chemical reaction is happening, and locate the carbon and hydrogen that are causing that reaction. High-quality crystals are essential to pinpointing these locations with accuracy and detail. If each molecule is like a face, high-quality crystals are crystals with all their faces beautifully aligned and looking in the same direction. This is why the microgravity environment of space is attractive.
In space, protein molecules can align beautifully because there is no convection, where aqueous solution moves vertically or horizontally due to a difference in concentration, nor sedimentation, where a heavy substance sinks due to the influence of gravity. So it is possible to form high quality crystals and observe protein structures in substantial detail.
Q. What kind of results have the Kibo experiments produced in the field of medicine?
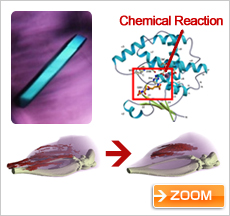
Protein involved in muscular dystrophy, and muscle atrophy reduced by the protein (courtesy: Dr. Yoshihiro Urade, Osaka Bioscience Institute)
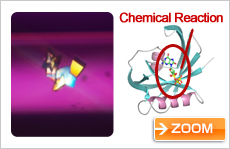
Protein involved in inhibition of cancer proliferation (courtesy: Prof. Yuriko Yamagata, Kumamoto University)

Protein expected to be used in bioenergy production (courtesy: Dr. Koji Inaka, Maruwa Foods and Biosciences, Inc)

Protein that degrades nylon oligomer (courtesy: Prof. Yoshiki Higuchi, University of Hyogo)
The closest to practical application is the research of Dr. Yoshihiro Urade of the Osaka Bioscience Institute. An effective treatment for muscular dystrophy is expected to be developed soon. Muscular dystrophy is a disease that weakens muscles to the point where they lose their function, and it had been thought to be incurable. However, by using the Kibo facility to form and analyze protein crystals connected with the progress of the disease, Dr. Urade’s team was able to learn some crucial new information. They have detected water molecules involved in the function of the protein. That is, they discovered not just the shape of the active site in the protein (the keyhole), but also the shape of the compound that binds to it (the key). I think this achievement was possible because the experiment was conducted in space, where high-quality protein crystals are easier to create.
In collaboration with the pharmaceutical industry, Dr. Urade has developed a compound that is an effective drug candidate for muscular dystrophy. The compound was administered to a dog that had been induced to develop muscular dystrophy, and the result was very favorable. Dr. Urade’s research continues, and he hopes to have the compound certified as a new medicine as soon as possible.
Other than this, Prof. Sam Yong Park of Yokohama City University is working on a protein involved in DNA replication, with the aim of developing breakthrough medication effective for influenza. Influenza outbreaks happen every winter, and so it is essential to have the right vaccine available that will target the prevailing type of influenza virus. Also, there is the increasing emergence of drug-resistant influenza viruses that are resistant to current medicines. Prof. Park has figured out the structure of the protein the causes DNA information to be replicated in various types of influenza virus. Studying the structure of the protein in detail will allow scientists to develop new medicine that inhibits the function of influenza viruses regardless of their type.
Finally, Prof. Yuriko Yamagata of Kumamoto University is studying the protein involved in cancer proliferation. The hope is that inhibiting the function of the protein could stop the proliferation of cancer, and reduce the size of cancer cells. Growing these protein crystals in space, Prof. Yamagata has been able to learn the details of their three-dimensional structure. Now, scientists are trying to find a compound (key) that will fit that keyhole.
Many other medical experiments are being carried out on Kibo, with growing hopes for practical application. Q. What other kinds of results have come out from experiments on Kibo? Dr. Koji Inaka of Maruwa Foods and Biosciences, Inc. has conducted research on bioenergy production. He has looked at the structure of an enzyme called cellulase, which breaks down plant cell walls and a protein that’s a main ingredient of fiber. Recently, there has been a focus on environmentally friendly bioenergy. However, the most common form, bioethanol, is made of chemically processed food crops, such as corn. Energy production with the cellulase enzyme does not require the use of food crops; instead it involves dissolving the plant fiber of grass, straw, timber and waste. If this becomes feasible, I think it will help solve Japan’s problem of food self-sufficiency. Instead of using food crops to produce energy, we’ll be able to use them as actual food. Dr. Inaka is aiming for practical application of the enzyme in three to five years.
Prof. Yoshiki Higuchi of the University of Hyogo is studying an enzyme that helps break down by-products of nylon (plastic) manufacture. These by-products, known as nylon oligomers and monomers, have conventionally been thrown away. In our daily life, we use a lot of artificial fiber and plastics, but they degrade naturally only with great difficulty, and result in a lot of waste we have to dispose of. If it becomes possible to make an enzyme that can efficiently break down this waste and turn it once more into a raw material, it will be a great contribution to the environment. The development of such an enzyme is currently underway not only for waste disposal purposes but also for industrial applications.