Space Experiments Provide the First Structural Information of the
Family S46 Peptidase which is a Novel Drug Target Against
Multidrug-resistant Bacteria and Periodontal Disease Bacteria.
- Discovery of Novel antimicrobial drug is expected -
May 16, 2014 (JST)
Iwate Medical University
Showa University
Nagaoka University of Technology
Japan Aerospace Exploration Agency
Key
- We determined the structures of DAP BII* for the first time as enzymes belonging to the S46 peptidase family, a peptidase having structural and functional similarities to important enzymes for the growth of multidrug-resistant bacteria and periodontal disease bacteria, using X-ray crystal structure analysis through high quality protein crystallization space experiments in the Japanese Experiment Module "Kibo" of the International Space Station.
- We clarified the conformation of a complex of DAP BII with degradation product and the mechanisms of the substrate recognition and the reaction, and observed a large-scale conformation change upon substrate binding.
-
It is expected that the findings of this study will help in the development of new antimicrobial drugs against multidrug-resistant bacteria causing difficult-to-treat hospital-acquired infections.
* DAP BII: A peptidase produced by Pseudoxanthomonas mexicana WO24.
A research group consisting of Yasumitsu Sakamoto, an Assistant Professor of the School of Pharmacy at Iwate Medical University, Nobutada Tanaka, an Associate Professor of the School of Pharmacy at Showa University, Wataru Ogasawara, an Associate Professor of the Department of Bioengineering at Nagaoka University of Technology, and Kazunori Ohta, an Associate Senior Engineer of Japan Aerospace Exploration Agency (JAXA), clarified the conformation and substrate recognition mechanism of a peptidase DAP BII, the structure and function of which are extremely similar to those of enzymes playing an important role in the peptide metabolism of multidrug-resistant bacteria and periodontal disease bacteria for the growth of these bacteria, by crystallizing DAP BII in the Japanese Experiment Module "Kibo" of the International Space Station (ISS) (Term 1) and performing X-ray crystal structure analysis of the obtained crystals.
Multidrug-resistant bacteria, which acquired resistance against a variety of antimicrobial drugs, is one of the causes of hospital-acquired infections, which are difficult to treat. The findings of this study provide us with an effective approach to develop novel antimicrobial drugs with a new mode of action against multidrug-resistant bacteria and periodontal disease bacteria. The results of this study were published in "Scientific Reports" (Nature Publishing Group) at 6:00 p.m. on May 15 at (Japan Standard Time).
Significance of study
Multidrug-resistant bacteria are bacteria with resistance against multiple antibacterial drugs. Multidrug-resistant bacteria are a menace for patients with compromised immune systems due to chemotherapy for cancer and diabetes and patients receiving mechanical ventilation because existing antibiotics fail to work. In addition, relationships of periodontal diseases with not only tooth disorders but also systemic diseases such as arteriosclerosis and diabetes have been suggested. Infectious diseases are often treated with antibiotics; therefore, antimicrobial drugs causing suppression of the growth or exerting bactericidal effects with the mechanisms different from existing antibiotics are required for antibiotic-resistant bacteria.
Some multidrug-resistant bacteria and periodontal disease bacteria are also called sugar nonfermentative bacteria (Term 2), which get energy mainly from amino acids obtained by degradation of protein and peptide instead of carbohydrates such as sugars. These bacteria secrete a variety of proteases and peptidases. Substances that prevent the function of these catabolic enzymes are called inhibitors. Chemical compounds that specifically bind with these enzymes become candidates for such inhibitors. Also it is important that humans have no enzyme similar to the target enzyme of the new antimicrobial drug because if humans have enzyme similar to the target enzyme the drug may develop side effects because of the inhibition of similar enzyme. The enzyme belonging to the S46 peptidase family, of which conformation was determined in this study, is known to have no similar enzymes in humans but the enzyme is important for the peptide metabolism in the sugar nonfermentative bacteria. Accordingly, inhibitors targeting at the S46 peptidase family have the potential for the development of novel antimicrobial drugs.
Summary of findings
The structure of DAP BII was clarified for the first time as an enzyme belonging to the S46 peptidase family. The conformation showed a totally new structure that was not known before. In addition, it is important for the development of inhibitors to understand the interaction between enzyme and chemical compounds. In this study, we succeeded to obtain the structures of DAP BII- degradation product complex, which are important for elucidation of such interaction.
We clarified the mechanisms of peptide degradation and substrate recognition from these conformations. Such structural information enables us to design inhibitors that specifically bind to the S46 peptide family. Furthermore, it was shown that the structures of DAP BII alone and DAP BII complex with a degradation product are markedly different. (Figures on the next page) After this, we are planning to target the development of new antimicrobial agents through design of inhibitors effective for pathogenic bacteria such as multidrug-resistant bacteria and screening of chemical compounds based on the findings of this study.
This study was conducted as part of high quality protein crystallization space experiments of JAXA, whose representative is Yasumitsu Sakamoto an Assistant Professor of the Department of Structural Biology, School of Pharmacy, Iwate Medical University (Head of the Department: Takamasa Nonaka). Crystallization under microgravity conditions in the "Kibo" of the ISS enabled to make a great improvement in the quality of DAP BII crystal and obtain the data with a resolution of 1.95 Å (Term 3) although the resolution of the existing crystallization on the earth was only 3.4 Å. The high-resolution data obtained in this space experiment greatly contributed to the elucidation of a conformation change caused by the binding of DAP BII and a peptide.
Furthermore, this study was conducted by collaboration among Nobutada Tanaka, an Associate Professor of the School of Pharmacy at Showa University; Wataru Ogasawara (from Iwate), an Associate Professor of the Department of Bioengineering at Nagaoka University of Technology; and Kazunori Ohta, an Associate Senior Engineer of JAXA with support from the Program for the Strategic Research Foundation at Private Universities; Grants-in-Aid for Scientific Research; Platform for Drug Discovery, Informatics, and Structural Life Science Project, etc.
Data collection was conducted using the Photon Factory of the High Energy Accelerator Research Organization (Tsukuba) and SPring-8, a large synchrotron radiation facility (Harima). The utilization of these facilities was essential for structural analyses.
Paper title: "S46 Peptidases are the First Exopeptidases to be Members of Clan PA"
Journal title: "Scientific Reports"
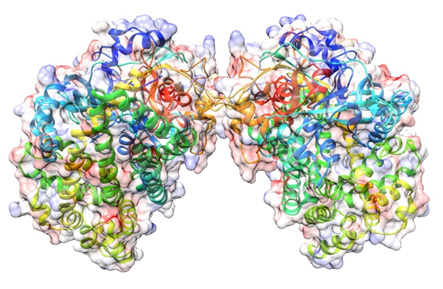 The entire structure of DAP BII |
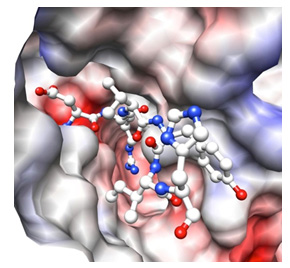 Binding of peptide to DAP BII |
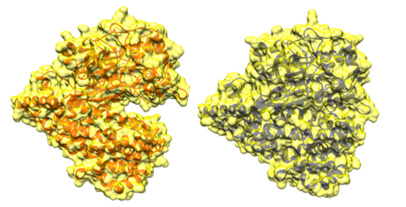 Conformation change of DAP BII |
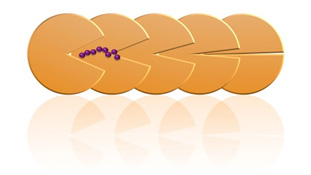 Binding with a peptide DAP BII and cleavage of the peptide |
Courtesy of Yasumitsu Sakamoto, Assistant Professor of the
School of Pharmacy at Iwate Medical University
Glossary
1. Japanese Experiment Module "Kibo" of the International Space Station (ISS):
Japan's first human space facility of the ISS, which is the largest space facility in the history of mankind constructed in space about 400 kilometers above the ground. The Kibo consists of two experiment facilities — the Pressurized Module and Exposed Facility — wherein scientific experiments using a microgravity environment and cosmic radiation, experiments using outer space for prolonged periods as well as astronomical observations and earth observations are conducted.
2. Asaccharolytic bacteria:
A classification category of bacteria based on their characteristics. Bacteria such as Escherichia coli, which get energy from the degradation of glucose, are categorized into fermentative bacteria, and bacteria, which get energy from degradation of protein and peptide, are categorized into the sugar nonfermentative bacteria. Some of the sugar nonfermentative bacteria are less sensitive to antibiotics because of secretion ofβ-lactamase that degrades antibiotics. Typical sugar nonfermentative bacteria with S46 peptidase that we focus on in this study include Stenotrophomonas maltophilia, which often develops multidrug-resistant bacteria, and Porphyromonas gingivalis and Porphyromonas endodontalis, which are periodontal disease bacteria.
3. Å (Angstrom):
Ten billionth part of a meter (10-10 m). The radius of a hydrogen atom, which is the smallest atom, is 1.2 Å.